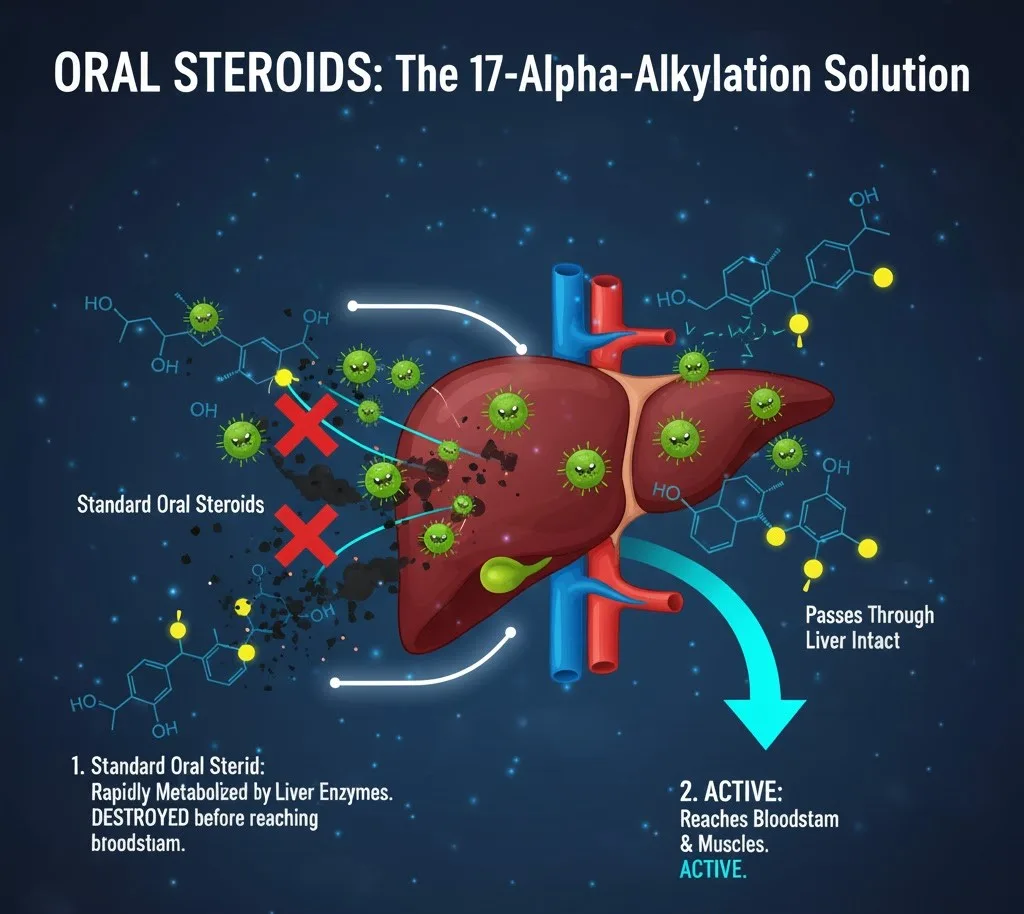
Oral steroids, particularly those intended to mimic or enhance the effects of testosterone, present a significant challenge in pharmacology. When a standard steroid is ingested, its journey through the digestive system inevitably leads it to the liver, the body's primary metabolic organ. Here, a critical issue arises: the liver's powerful enzymes rapidly metabolize and break down the steroid compound.
The Metabolic Dilemma:
The core problem is that this rapid metabolism often leads to the destruction of the active compound, such as testosterone, before it can even reach the systemic circulation (the bloodstream) and subsequently the target tissues like muscles. In essence, the therapeutic potential of the oral steroid is largely nullified, as very little, if any, of the beneficial compound survives this "first-pass metabolism" through the liver. This inefficiency makes plain, unmodified testosterone ineffective when taken orally.
The Pharmaceutical Solution: 17-Alpha-Alkylation
To overcome this metabolic hurdle, pharmaceutical scientists developed a clever structural modification known as 17-Alpha-Alkylation. This involves adding an alkyl group (typically a methyl or ethyl group) at the 17th carbon position of the steroid molecule.
How 17-Alpha-Alkylation Works:
This seemingly minor chemical alteration has a profound effect on the steroid's interaction with liver enzymes. The added alkyl group at the 17-alpha position creates steric hindrance, essentially making it more difficult for the liver's metabolic enzymes (particularly 17-beta-hydroxysteroid dehydrogenase}$) to bind to and break down the steroid.
By making the steroid resistant to this enzymatic degradation, 17-alpha-alkylation allows a significantly larger proportion of the orally administered steroid to pass through the liver intact. This preserved compound can then enter the bloodstream and exert its desired effects on muscles and other target tissues throughout the body.
Implications and Considerations:
While 17-alpha-alkylation has been crucial in developing effective oral anabolic steroids, it's important to note that this modification is also associated with a significant increase in liver toxicity. Because the liver struggles to metabolize these altered compounds, they can place a considerable strain on hepatic function, potentially leading to cholestasis, hepatitis, and in severe cases, liver damage.
Therefore, while 17-alpha-alkylation brilliantly solves the problem of oral bioavailability for steroids, it introduces a critical safety consideration that necessitates careful medical supervision and monitoring when such compounds are used. This highlights the delicate balance between efficacy and safety in drug design.